A Hydrolysis Reaction Results in Which Two of the Following
CH 3 CO 2 H H 2 O CH 3 CO 2- H 3 O. Identify the following reactions as condensation or hydrolysis Sort these items into the proper categories.

Hydrolysis Reaction Of Vegetable Oil Download Scientific Diagram
O The best answer is A H2O B C.

. Water is used in the reaction to break down A making it a hydrolysis reaction. Enter the missing materials according to the following format. The hydrolysis reaction of polymerspolypeptidesbreaks them down into monomers that areamino acidsWater is added and covalentpeptide bondsbetween amino.
Strong acid-weak base salt hydrolysis where the salt cation yields a proton to water to form a hydronium ion H3O. 0085 i Calculate the average rate of reaction between the time interval 30 to 60 seconds. Hydrolysis of acids and bases.
Acid-base hydrolysis where water splits into a hydroxyl ion OH and a proton H. A chemical bond is cleaved and two new bonds are formed each one having either the hydrogen component H or the hydroxyl component OH of the water molecule. Ii Calculate the pseudo first order rate constant for the hydrolysis of ester.
D the reaction of a fat forming glycerol and fatty acids with the utilization of water. 90 Estermol L - 1. Hydrolysis h aɪ ˈ d r ɒ l ɪ s ɪ s.
General formula of a hydrolysis reaction is. Hydrolysis of the haloalkane shown below results in the formation of two alcohols B and C that are stereoisomers of each other. Stores the energy that has been converted from food Reactants.
Then bromination is carried out in presence of potassium hydroxide which leads to formation of the preoduct as follows. Give the structures of the alcohols B and C and propose a mechanism for their formation ensuring that you explain the stereochemical outcome observed. Pressing New Reaction will display a partial hydrolysis reaction with two species shown and two missing.
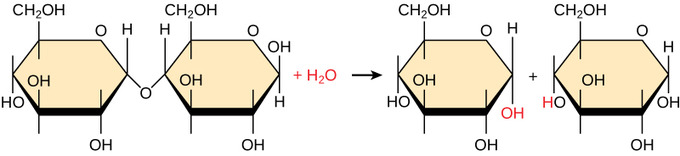
A reaction in which two molecules combine forming one and a molecule of water is produced the breaking of a glycosidic bond a disaccharide breaking into two monosaccharides Hydrolysis Condensation Identify disaccharides that fit each of the. Then the frequency of the fundamental note will be. It is toxic reacts with water.
Correct option is D in the initial step of the reaction thionyl chloride is used which acts as a chlorinating reagent with chemical formula of SoCl 2. The frequency of the fundamental note in a wire stretched under tension T is f. There are different forms of hydrolysis depending on the substances that are made to react with water.
The cellobiose can then be further hydrolyzed to create glucose. A chemical reaction using water to separate molecules Adenosine Tri-Phosphate ATP. Thus the lysis of fat into glycerol and fatty acids in the presence of.
Hydrolysis reactions were carried out at pH 80 with 10 min TAG 15 mmol were emulsified mechanically in 40 mL of incubation at various temperatures 22 35 45 50 55 60 of NaCl solution 015 M. A the reaction of two monosaccharides forming a disaccharide with the release of water B the reaction of a fat with glycerol forming fatty acids with the release of water C the synthesis of two amino acids forming a peptide bond with the release of water. Hydrolysis of cellulose an organic compound used to make all sorts of things from paper to biofuels results in a split in the chemical bonds between cellobiose and cellulose.
The hydrolysis reaction of polymerspolysaccharidesbreaks them down into monomersmonosaccharidesWater is added and covalentglycosidic bondsbetween monosaccharides are broken. Hydrolysis of the haloalkane shown below results in the formation of two alcohols B and C that are stereoisomers of each other. HI H 2 O I - H 3 O.
Thus a hydrolysis reaction is the cleavage of chemical bonds by the addition of water or a base that supplies the hydroxyl ion OH. Which of the following is an example of hydrolysis. Hydrolysis is defined as the break down of a chemical compound because of the action of water.
The frequency 1057 MHz of radiation arising from two close energy levels in hydrogen belongs to. The function a sinx b cos x c sin x d cos x is decreasing if. AH H 2 O A - H 3 O Ex.
The term comes from the Greek prefix hydro - water and lysis to break apart. In a pseudo first order hydrolysis of ester in water the following results were obtained. Give two examples to show the.
Given what you now know what information andor problem solving approach is most likely to produce the correct answer. The term is used broadly for substitution elimination and solvation reactions in which water is the nucleophile. Enter the missing materials according to the following format.
Question 1 Which of the following is a hydrolysis reaction. The reaction of two monosaccharides to form a disaccharide O a the reaction of two amino acids to form a dipeptide O the reaction of a hydrogen atom and a hydroxide ion to form water O the reaction of a fat to form glycerol and fatty acids Question 2 Which of the following molecules is a. AB H 2 O AH BOH.
Biological hydrolysis is the cleavage of biomolecules a water molecule is. The acid hydrolysis of the ester is. From Ancient Greek hydro- water and lysis to unbind is any chemical reaction in which a molecule of water breaks one or more chemical bonds.
The molecules involved in initial. If the tension is increased to 25 T. A the reaction of two monosaccharides forming a disaccharide with the release of water B the synthesis of two amino acids forming a peptide with the release of water C the reaction of a fat forming glycerol and fatty acids with the release of water D the reaction of a fat forming glycerol and fatty acids with the consumption.
Carboxylic Acids end in CO2H. H3CH2C CH3 H20 heat В с Br. Which of the following is an example of a hydrolysis reaction.
I first order reaction ii bimolecular reaction iii unimolecular reaction iv second order reaction. Give the structures of c the alcohols B and C and propose a mechanism for their formation ensuring that you explain the stereochemical outcome observed. Hydrolysis may be considered the reverse of a condensation reaction in which two molecules combine with each other producing water as one of the products.
Pressing New Reaction will display a partial hydrolysis reaction with two species shown and two missing. Hydrolysis reactions were carried and 68C. HI H 2 O I - H 3 O.
In this case the reactant A is being broken down to form B and C. HA H 2 O A - H 3 O Ex. HA H 2 O A - H 3 O Ex.

Hydrolysis Reaction Of Vegetable Oil Download Scientific Diagram

No comments for "A Hydrolysis Reaction Results in Which Two of the Following"
Post a Comment